whats a good buffer to use for ph 8
 Every day scientists in laboratories across the globe sit at their desks and painstakingly design experiments in the hope of making a discovery that volition change how we remember about a biological process. Because biological processes such as enzymatic activity are dependent on pH, one critical aspect of the experimental design is choosing a buffering system that will help maintain a stable pH without altering the results.
Every day scientists in laboratories across the globe sit at their desks and painstakingly design experiments in the hope of making a discovery that volition change how we remember about a biological process. Because biological processes such as enzymatic activity are dependent on pH, one critical aspect of the experimental design is choosing a buffering system that will help maintain a stable pH without altering the results.
And, ofttimes, it is the choice of buffer that makes or breaks the experiment. It is possible that the buffer you are using in your lab might be the reason your experiment is failing. Hither, y'all will find how a buffering system works, a description of the characteristics of a good buffer and a listing of possible applications and characteristics of the most normally used biological buffers.
What is a buffer?
A buffer consists of a weak acid (proton donor, HA) and its conjugate base (proton acceptor, A -). In water, HA tin dissociate into A- and H+. H+ and so reacts with water to course H3O+. In the aqueous buffer solution, HthreeO+, HA and H+ exist in equilibrium with each other. The buffering mechanism consists of two reversible reactions where the concentration of proton donor and proton acceptor are equal.
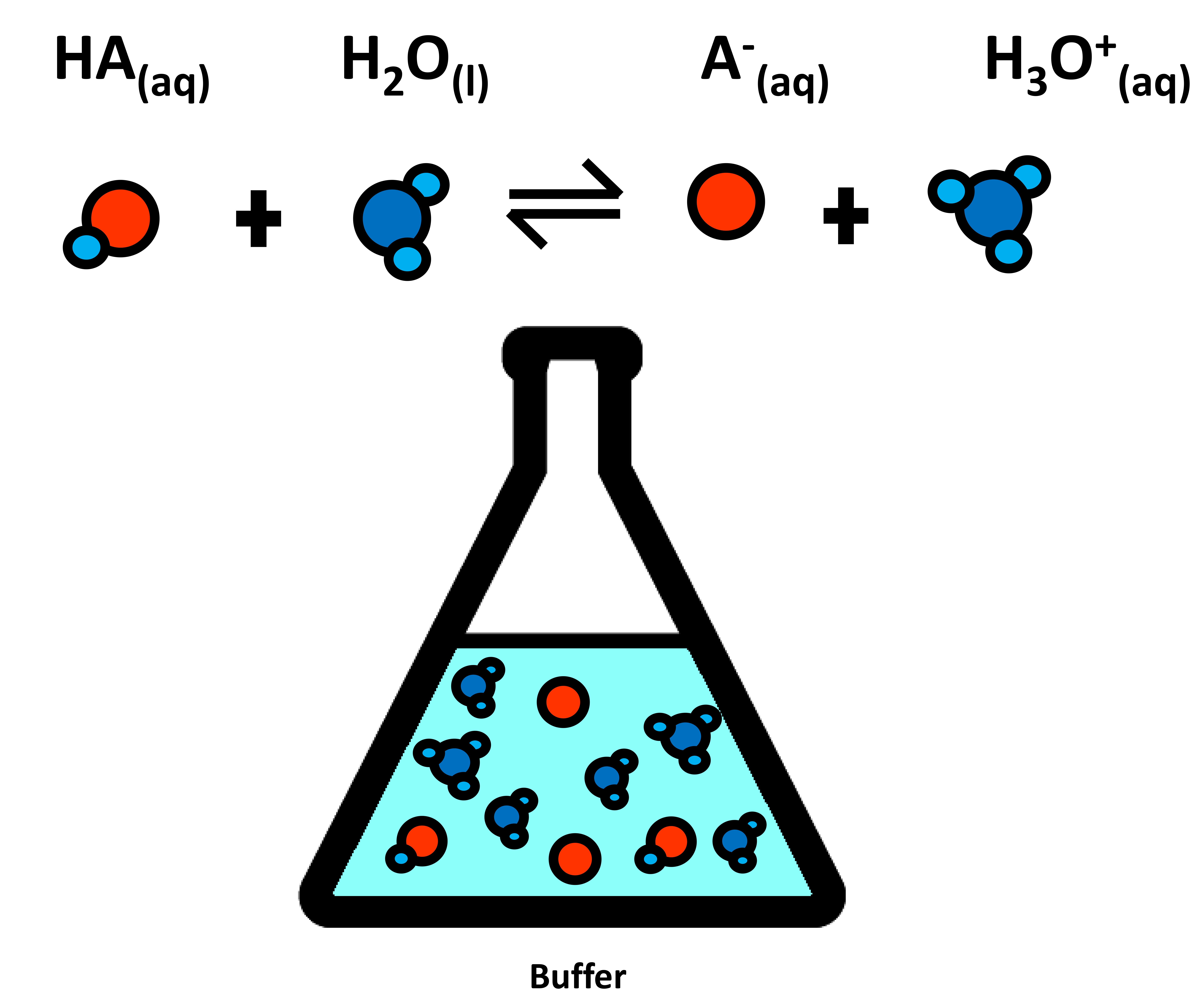
And so, when a strong acid or base is introduced into this system by the scientist or by enzymatic action during the experiment, the new ions from the introduced acid or base (H + or OH-) are absorbed past the buffer and the pH remains stable preventing changes in protein structure and role.
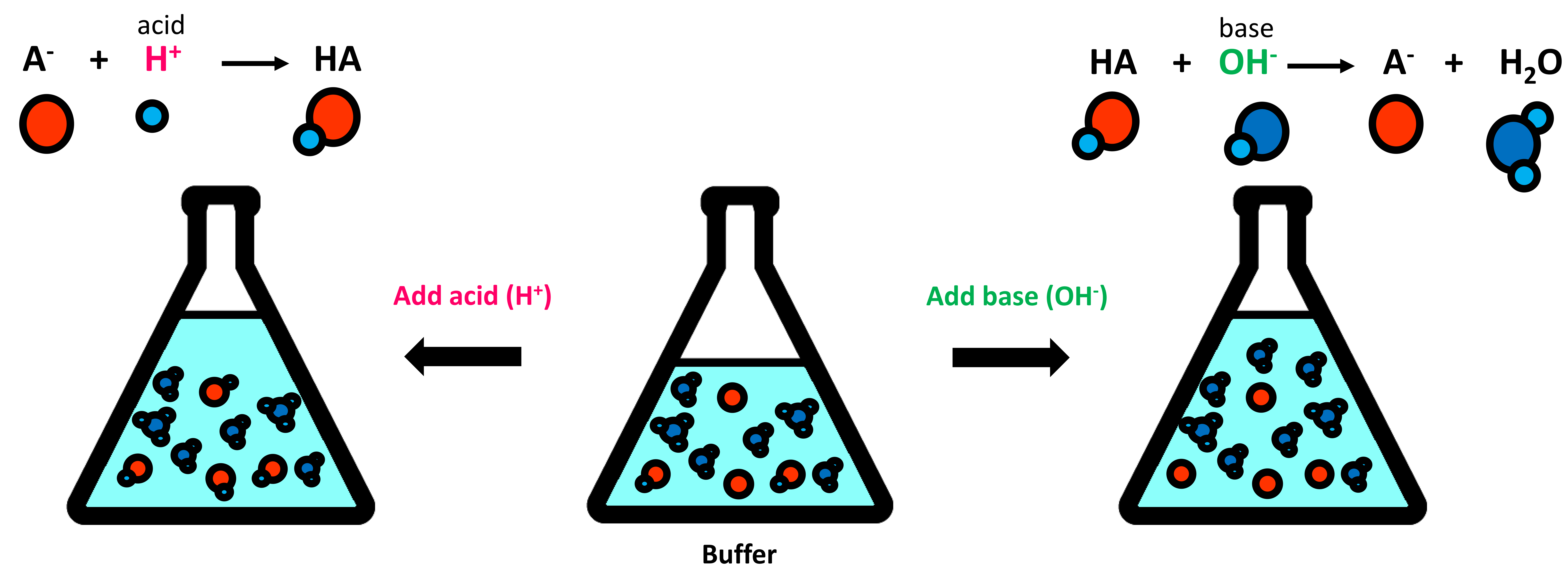
Buffers cannot arbitrarily moderate whatever changes in ion concentration. Their optimal buffering capacity, or range, is defined by the dissociation constant, or ka, of the acid. We unremarkably discuss buffering chapters in terms of the pKa or the logarithmic constant of ka. We consider the buffering capacity of a specific buffer to be the pKa ± 1. For example, a buffer with a pH of six.8 has a pH buffering range of 5.eight-7.eight.

What is a Good biological buffer?
Years ago, scientists performed biochemical experiments with inadequate buffers that greatly limited the touch on of their inquiry. These buffers exhibited high prison cell toxicity and could not support enzymatic activity throughout the procedures. Then, in 1966 Norman E. Skillful and his squad designed a series of buffers specifically for biological research with the following characteristics:
- Buffers should have a pKa between 6.0 and viii.0 because the optimal pH for most biological reactions rests in this range.
- Buffers should have high h2o solubility and minimum solubility in organic solvents so it remains in the aqueous medium of the biological system.
- Buffers should not permeate prison cell membranes. The buffer should not accumulate in cellular organelles. This may non use to your specific experiment. Zwitterionic buffers practice not permeate cell membranes.
- Buffers should have minimal salt effects because ionic buffers tin be problematic if the biological organization being studied is negatively afflicted by salts.
- The buffer's concentration, temperature and ionic composition of the medium should accept a minimal result on buffering capability (pKa).
- The germination of complexes between a metal ion and the buffer results in proton release, which affects the pH of the arrangement and may take an adverse issue on experimental results. Thus, these ionic complexes should exist soluble and their binding constant must be known. A buffer with a depression metal-bounden abiding is suitable for the study of metallic-dependent enzymatic reactions. If your experimental design requires the use of a metal, and then y'all should cull a buffer that does not form a complex with that specific metal.
- Buffers should exist stable and resist enzymatic and nonenzymatic degradation. And they should not interfere with enzyme substrates or resemble them.
- Buffers should not absorb light in the visible or ultraviolet regions of the spectrum to prevent interference in spectrophotometric assays.
- Their preparation and purification should exist piece of cake and inexpensive.
What are the most common buffers and how are they used?
The characteristics considered by Good and his team are a good starting point when choosing the buffer for your specific experiment. Here, we are including a Biological Buffer Choice Guide (click hither or curlicue downwardly for the PDF) containing a list of the most common biological buffers and the specific techniques and experiments they are used for. We are also including a description of their properties including pH, buffering range, metal binding capabilities, advantages and disadvantages and links to the protocols for the stock solutions.
In improver, we are including a Guide (click hither or scroll down for the PDF) of the most commonly used buffers in the lab describing their pH and composition.
Additional resource:
User guide for GoldBio buffers
GoldBio buffer nautical chart, by family
GoldBio buffer chart, past pH

References
10X Running buffer. (1970, January 01). Retrieved September 21, 2018, from http://cshprotocols.cshlp.org/content/2006/one/pdb.rec10475.full?text_only=true.
Ferreira, C. M., Pinto, I. S., Soares, Eastward. V., & Soares, H. M. (2015). (United nations)suitability of the utilize of pH buffers in biological, biochemical and ecology studies and their interaction with metallic ions – a review. RSC Advances, 5(39), 30989-31003. doi:10.1039/c4ra15453c.
Practiced, Northward., & Izawa, S. (1972). [iii] Hydrogen ion buffers. Methods in Enzymology Photosynthesis and Nitrogen Fixation Part B, 53-68. doi:10.1016/0076-6879(72)24054-ten.
Good, Northward. Due east., Winget, G. D., Wintertime, Due west., Connolly, T. N., Izawa, Due south., & Singh, R. Chiliad. (1966). Hydrogen Ion Buffers for Biological Research*. Biochemistry, 5(2), 467-477. doi:10.1021/bi00866a011.
Laboratory Stock Solutions and Equipment. (1998). Current Protocols in Prison cell Biology, 00(1). doi:ten.1002/0471143030.cba02as00.
Purich, D. L. (2010). Factors Influencing Enzyme Activity. Enzyme Kinetics: Catalysis & Control, 379-484. doi:10.1016/b978-0-12-380924-7.10007-9.
Tris-Glycine/MeOH Transfer Buffer. (1970, January 01). Retrieved September 21, 2018, from http://cshprotocols.cshlp.org/content/2015/five/pdb.rec087064.full?sid=3b0abcbe-5bcf-460f-87d8-48a5525a9d25.
SDS-Page Running Buffer. (1970, Jan 01). Retrieved September 21, 2018, from http://cshprotocols.cshlp.org/content/2014/7/pdb.rec081117.full?sid=f680f5fc-664e-4fe1-a840-07cec720d52e.
Zbacnik, T. J., Holcomb, R. E., Katayama, D. S., Tater, B. Grand., Payne, R. W., Coccaro, R. C., . . . Manning, Chiliad. C. (2017). Part of Buffers in Protein Formulations. Journal of Pharmaceutical Sciences, 106(iii), 713-733. doi:10.1016/j.xphs.2016.11.014.
Category Lawmaking: 79105, 79104, 88251
Biological Buffer Selection Guide
Guide: Unremarkably used Buffers
Fernanda Ruiz is a science content writer at Aureate Biotechnology. She holds a available's of science in biology from St. Mary's University and a PhD in molecular biology from Baylor College of Medicine.
Source: https://www.goldbio.com/articles/article/what-is-a-biological-buffer-and-how-to-choose-the-best-buffer-for-your-experiment
0 Response to "whats a good buffer to use for ph 8"
Post a Comment